Colloids
Colloids are of interest as directly visualizable molecular analogues on the micro- and nanoscale owing to their larger size and possession of Brownian motion. The Weck group develops methods to expand synthetic routes for the synthesis of anisotropic particles comprised of a variety of materials for use in various directed assembly methods. We are particularly interested in anisotropic colloids because unlike their spherical counterparts, they are capable of assembling into structures more advanced than the close-packed lattice. Directed assembly of our anisotropic particles is achieved using different approaches, including depletion forces, capillary forces, electric fields, and DNA hybridization (or a combination) to achieve open-packed structures. These structures are evaluated using various imaging instruments, including bright-field microscopy, confocal imaging and scanning electron microscopy. Quantitative analysis of images of these structures are performed using custom code the Weck group develops. Overall, we collaborate to combine our expertise in colloidal synthesis, assembly, and analysis to use bottom-up approaches to expand domain knowledge in the colloidal field.
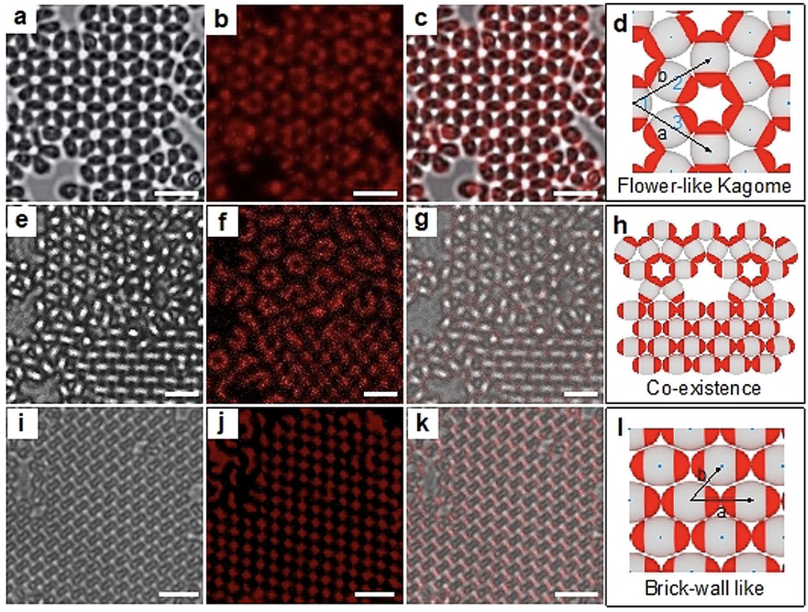
Patchy particles introduce directional bonding to the colloidal world and can be viewed as analogous to atoms and molecules. In order to expand the assemblies generated, the Weck group has developed a facile method for the synthesis of orthogonally functionalizable patchy particles, where all components of the particles are tunable. These particles are then used in a variety of different assembly conditions to generate various assemblies. The Weck group has published the assembly of various patchy particles into different superstructures, including chains and lattices of various symmetry.
Colloidal clusters with distinct structure and symmetry have been synthesized through the assembly of colloidal spheres via various self-assembly techniques, not limited to external fields and physical confinement. These clusters exhibit a vast range of applications and potential functions as artificial cells for drug transport and as building blocks for the assembly of 2-D and 3-D structures at the micron scale. The Weck group has published a synthetic strategy for fabricating multicomponent colloidal clustered molecules with controllable, complex morphologies that possess compositionally distinct lobes exhibiting chirality.
Supported Catalysis
Inspired by the concept of compartmentalization that Nature uses to coordinate thousands of incompatible chemical transformations into streamlined metabolic processes, the Weck group is developing micelles with orthogonal domains that can be used as nanoreactors for incompatible cascade transformations. The micelles are synthesized via the aqueous self-assembly of amphiphilic block copolymers. By functionalizing each block with a variety of catalysts we are able to carry out non-orthogonal chemical transformations in aqueous media with high conversions, selectivities, and catalyst recyclabilities. In particular, we are studying shell cross-linked micelles (SCMs) and multicompartment micelles (MCMs) as support structures.
Shell cross-linked micelles possess a hydrophobic pocket that provides an ideal microenvironment for the diffusion of small hydrophobic molecules. SCMs possess three orthogonal nanodomains: a hydrophobic core, a hydrophilic solubilizing corona, and a crosslinked block for nanostructure stability enabling catalysis in dilute aqueous environments. Each of these layers can be used for catalyst isolation enabling incompatible tandem reaction sequences to occur. Past work in the group has reported up to three transformations in one-pot. In efforts to regulate catalysis, “smart” SCMs have also been developed by the Weck group by incorporating responsive elements into the cross-linked layer to control substrate/reagent diffusion in and out of the micelle core.
We are also developing functionalized multicompartment micelles as nanoreactors for multistep step catalytic tandem reactions. Unlike a “traditional” micelle which has a hydrophilic corona and a single hydrophobic core, a multicompartment micelle has multiple microphase separated domains within its core, a result of the self-assembly of multiblocks copolymer with three or more mutually incompatible polymer blocks. The Weck group has synthesized linear and bottlebrush tri- and tetra-block copolymers with functionalized hydrophilic, lipophilic, siliphilic, and fluorophilic blocks. These polymers self-assemble in an aqueous solution to form functionalized multicompartment micelles, a new kind of nanostructure with tremendous potential in tandem catalysis.
Supramolecular Polymers
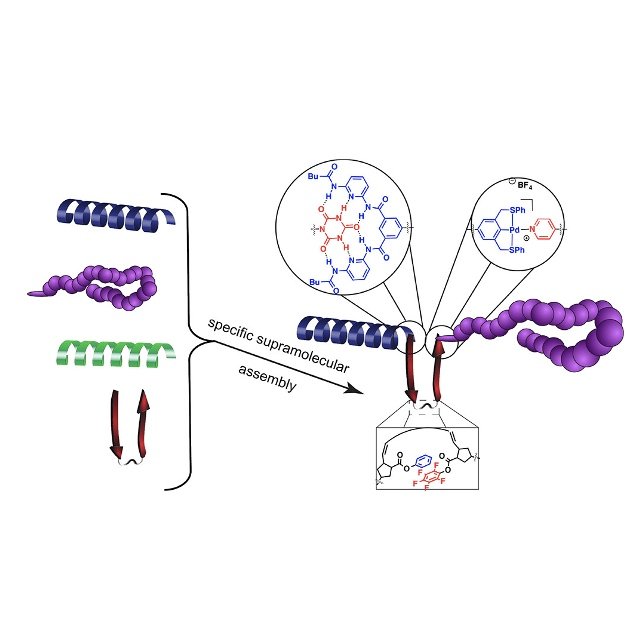
Protein’s structure and function have inspired chemists for decades. Their hierarchical structures contain a level of complexity—secondary and tertiary structures—that dedicates and diversifies protein functions. The bottom-up synthesis of protein-mimicking polymers mastering sequence variability and dispersity remains challenging. The Weck group’s approach centers on incorporating polymers with predefined secondary structures, such as helices and π–π stacking sheets, into block copolymers thereby circumventing the issue of designing and predicting one facet of a 3D architecture.
We have developed strategies toward block copolymers composed of non-amide based units bearing well-defined secondary structures mixed in a “plug-and-play” manner that approaches a modicum of the versatility seen in nature. For our building blocks we use ring-opening metathesis polymerization (ROMP) of [2.2]paracyclophane-1,9-dienes (pCpd), which forms conjugated poly(p-phenylenevinylene)s (PPVs) evocative of β-sheets. Helical building blocks arise from polymers such as poly(isocyanide)s (PICs) and poly(methacrylamide)s (PMAcs) containing bulky, chiral side groups while the coil motif can be represented by any flexible chain; we frequently choose poly(styrene) (PS) or poly(norbornene) (PNB). We install supramolecular interacting motifs at the chain-ends or side-chains of our building blocks for orthogonal assemblies creating tertiary-like structures. Our studies demonstrate the retention of distinct architectures after complex assembly, a paradigm that we believe may extend to other polymeric folding systems. Ongoing and future work focus on using orthogonal assembly strategies and available building blocks to create 3D hierarchical architectures that emulate the structural complexity and functionality of proteins.
Biomaterials
The Weck group is developing new methods for the rapid synthesis of biomaterials. We are developing new polymeric scaffolds for bone tissue engineering by combining: (i) a strategy to synthesize tunable and highly functionalized block copolymers, (ii) crosslinking of a nano/mesoscale separated phase to enhance mechanical properties, (iii) processing to provide a reinforced porous scaffold, and (iv) incorporation of biomimetic ligands to support osteoblast adhesion and function. The combination of living polymerization techniques and thermally induced phase separation allows us to address the issues of inadequate mechanical properties and the lack of control of scaffold architectures. In particular, our strategy is based on poly(lactic acid)/poly(norbornene) (PLA/PNB) block copolymers that are synthesized through a combination of ring-opening polymerization of lactides with ROMP, followed by the foaming of the resulting block copolymers into highly porous scaffolds. PLA is a biodegradable and biorenewable polymer that has been used extensively in a variety of biomedical applications. The Weck group has reported the first synthetic approaches towards functionalized poly(lactic acid) including the attachment of PEG and peptide sequences onto PLA.
A second thrust area in biomaterials is the development of delivery vehicles forin vitroandin vivoimaging and delivery applications. The Weck group is developing a straight-forward methodology for the synthesis of multifunctionalized linear polymers and dendrimers based on an orthogonal functionalization strategy in close analogy to the noncovalent methodology. For example, polyamide-based dendrimers possessing both a aldehyde, azide and amine moieties on their periphery have been synthesized and functionalized quantitatively with small organic and biological molecules with complete specificity of each functional moiety for its complementary motif. This orthogonal functionalization strategy has the potential to synthesize multifunctional dendrimers for a variety of applications ranging from targeted biological delivery vehicles to optical materials.